Cu375 / 375 SL
This model is completely identical with the long-term tried and trusted bestseller "Multiload Cu 375/375 SL".
Insertion requires a one-hand technique only without preloading.
This Mona Lisa model is available in two versions:
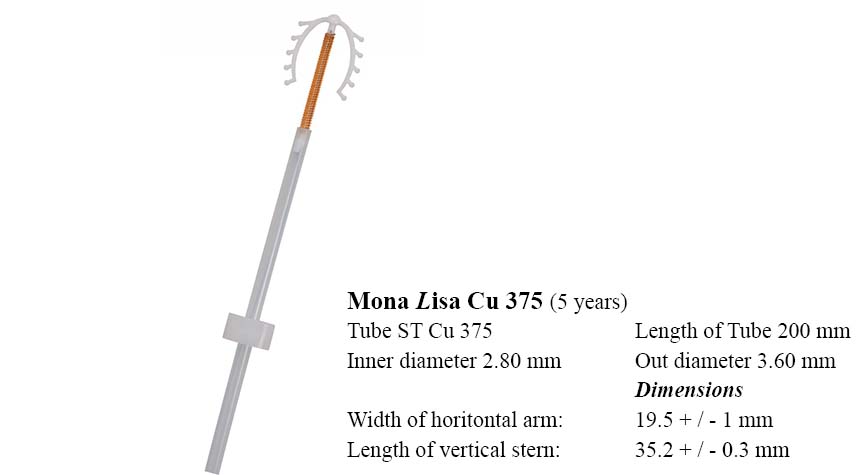
Cu375
With a copper surface of 375 mm² this version guarantees 5 years of safe contraception.
Unique features:
- One-hand technique for insertion
- low expulsion rate due to the flexible IUD-body
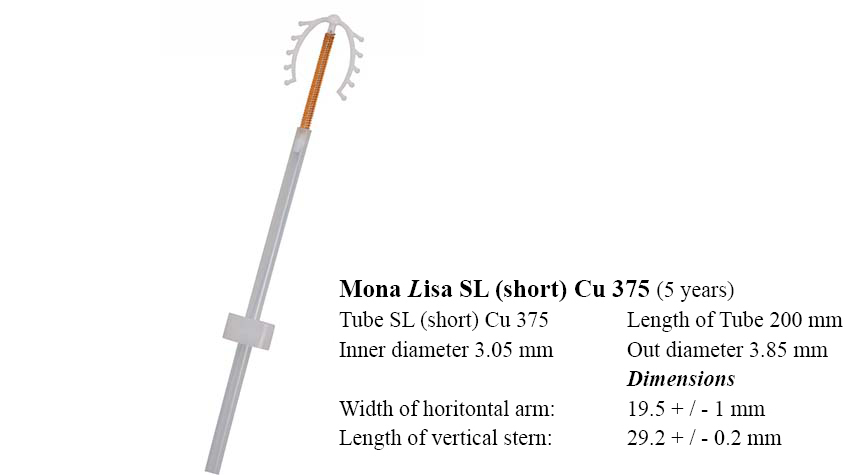
Cu375 SL
This version is identical to the Mona Lisa Cu 375,only differing in the dimension.
Unique features:
- the stem length (29,4 mm) is 5 mm shorter compared to the Mona Lisa Cu375
- ideally suited for patients with shorter uterus length
FAQ's
It is not fully known how IUDs work. However, nowadays it is assumed that the most probable effect is that it disrupts the normal function of the male gametes (sperm), which become incapable of fertilising the female egg. It is also assumed that copper ions influence the development of the egg so that fertilization doesn´t take place: IUDs are no longer considered a method of abortion.
Insertion is recommended during or shortly following menstruation. If pregnancy is excluded, Mona Lisa IUDs may be inserted at any time of the cycle. Postpartum insertion should be postponed until six weeks after delivery, as this is associated with high rates of perforation and expulsion. There are no doubts against breast feeding at lying IUD.
The available experience indicates that, in general, drugs interfering with contraceptive efficacy of Mona Lisa IUDs are highly unlikely. However, published reports appear to show diminished efficacy in the presence of long-term use of non-steroidal anti-inflammatory drugs (especially acetylsalicylic acid) and corticoids. Short-term use in the treatment of dysmenorrhoea with not-steroidal anti-inflammatory drugs does not appear to reduce contraceptive efficacy.
Mona Lisa IUDs may not be used at:
- Malignant diseases of the genital tract
- Vaginal bleeding
- Pregnancy
- Past history of ectopic pregnancy or predisposing factors
- infections of the genital tract
- Sexually transmitted diseases during the last 12 months (except bacterial vaginitis, repeated herpes infection, hepatitis B)
- Abortion with infection during the last 3 months, pelvic inflammatory disease (PID)
- Uterine malformations (congenital or acquired)
- Allergy to copper
MR Conditional
Non-clinical testing has demonstrated that the Mona Lisa intrauterine devices are MR condition. A patient with a Mona Lisa intrauterine device can be safely scanned in a MR system with a static magnetic field up to 3 Tesla.
The signed Declaration of the manufacturer is available under section "Quality System".
NT Cu380 / 380 Mini
This Mona Lisa model has the same design as the "Nova T 380".
Also this model requires preloading before insertion. In contrast to the CuT 380A version, the horizontal arms are to be pulled into the tube.
This Mona Lisa model is available in two versions:
NT Cu380
With a copper surface of 380 mm², this IUD model guarantees safety up to 5 years.
Unique features:
- Simple handling
- Easy and painless insertion and removal
- Low PID (Pelvic inflammatory disease) rate
NT Cu380 Mini
CThe NT Cu380 Mini has identical features like the NT Cu380 and differs only in the dimension from the standard model.
Unique features:
- less stem length as well as less width of the side arms compared to the NT Cu380
- ideally suited for women with a uterus length < 7 cm
FAQ's
It is not fully known how IUDs work. However, nowadays it is assumed that the most probable effect is that it disrupts the normal function of the male gametes (sperm), which become incapable of fertilising the female egg. It is also assumed that copper ions influence the development of the egg so that fertilization doesn´t take place: IUDs are no longer considered a method of abortion.
Insertion is recommended during or shortly following menstruation. If pregnancy is excluded, Mona Lisa IUDs may be inserted at any time of the cycle. Postpartum insertion should be postponed until six weeks after delivery, as this is associated with high rates of perforation and expulsion. There are no doubts against breast feeding at lying IUD.
The available experience indicates that, in general, drugs interfering with contraceptive efficacy of Mona Lisa IUDs are highly unlikely. However, published reports appear to show diminished efficacy in the presence of long-term use of non-steroidal anti-inflammatory drugs (especially acetylsalicylic acid) and corticoids. Short-term use in the treatment of dysmenorrhoea with not-steroidal anti-inflammatory drugs does not appear to reduce contraceptive efficacy.
Mona Lisa IUDs may not be used at:
- Malignant diseases of the genital tract
- Vaginal bleeding
- Pregnancy
- Past history of ectopic pregnancy or predisposing factors
- infections of the genital tract
- Sexually transmitted diseases during the last 12 months (except bacterial vaginitis, repeated herpes infection, hepatitis B)
- Abortion with infection during the last 3 months, pelvic inflammatory disease (PID)
- Uterine malformations (congenital or acquired)
- Allergy to copper
MR Conditional
Non-clinical testing has demonstrated that the Mona Lisa intrauterine devices are MR condition. A patient with a Mona Lisa intrauterine device can be safely scanned in a MR system with a static magnetic field up to 3 Tesla.
The signed Declaration of the manufacturer is available under section "Quality System".


CuT 380A QL
This model is an improved rebuilt of the former, no more available “GyneT” with a new technology for quick and safe loading of the IUD.
The classic T-shaped IUD is the most common used IUD-model in the world, recommended even by the WHO. With a copper surface of 380 mm² it shows the lowest pearl index among all copper IUDs.
Mona Lisa CuT 380A QL convinces through secure fit in the uterus and in consequence low expulsion rate because of optimal memory test results.
Before insertion, the IUD has to be preloaded by using the plunger. Due to the development of the insertion body, loading of the IUD is possible in seconds.
Unique features:
- The Mona Lisa CuT 380A has a copper surface of 380 mm² and guarantees safe contraception up to 10 years (recommendation of the WHO)
- The copper sleeves are completely embedded in the horizontal arms. This results in a very smooth and plane surface
- The longer insertion tube of the Mona Lisa CuT380A QL improves positioning of the IUD with patients with a longer uterine cavity
- Effortless and quick loading of the IUD because of the new insertion body
FAQ's
It is not fully known how IUDs work. However, nowadays it is assumed that the most probable effect is that it disrupts the normal function of the male gametes (sperm), which become incapable of fertilising the female egg. It is also assumed that copper ions influence the development of the egg so that fertilization doesn´t take place: IUDs are no longer considered a method of abortion.
Insertion is recommended during or shortly following menstruation. If pregnancy is excluded, Mona Lisa IUDs may be inserted at any time of the cycle. Postpartum insertion should be postponed until six weeks after delivery, as this is associated with high rates of perforation and expulsion. There are no doubts against breast feeding at lying IUD.
The available experience indicates that, in general, drugs interfering with contraceptive efficacy of Mona Lisa IUDs are highly unlikely. However, published reports appear to show diminished efficacy in the presence of long-term use of non-steroidal anti-inflammatory drugs (especially acetylsalicylic acid) and corticoids. Short-term use in the treatment of dysmenorrhoea with not-steroidal anti-inflammatory drugs does not appear to reduce contraceptive efficacy.
Mona Lisa IUDs may not be used at:
- Malignant diseases of the genital tract
- Vaginal bleeding
- Pregnancy
- Past history of ectopic pregnancy or predisposing factors
- infections of the genital tract
- Sexually transmitted diseases during the last 12 months (except bacterial vaginitis, repeated herpes infection, hepatitis B)
- Abortion with infection during the last 3 months, pelvic inflammatory disease (PID)
- Uterine malformations (congenital or acquired)
- Allergy to copper
MR Conditional
Non-clinical testing has demonstrated that the Mona Lisa intrauterine devices are MR condition. A patient with a Mona Lisa intrauterine device can be safely scanned in a MR system with a static magnetic field up to 3 Tesla.
The signed Declaration of the manufacturer is available under section "Quality System".

ST Cu300
The Mona Lisa ST Cu300 is a rebuilt of the Flexi T 300 which has been on the market since a long time. At the lower end of the IUD a secure double nylon thread system is fixed to the IUD body. The CU-Safe T 300 has a copper surface of 300 mm² and guarantees safe contraception up to 5 years.
The ST Cu300 has not to be preloaded; insertion takes place with a one-hand technique.
Unique features:
- Optimally suited for Nullipara, but also for Uni- and Multipara with a small uterus size
- Easy and pain free insertion and removal due to the small size of the IUD
- Low expulsion rate due to the shape optimally fitted to the uterus
FAQ's
It is not fully known how IUDs work. However, nowadays it is assumed that the most probable effect is that it disrupts the normal function of the male gametes (sperm), which become incapable of fertilising the female egg. It is also assumed that copper ions influence the development of the egg so that fertilization doesn´t take place: IUDs are no longer considered a method of abortion.
Insertion is recommended during or shortly following menstruation. If pregnancy is excluded, Mona Lisa IUDs may be inserted at any time of the cycle. Postpartum insertion should be postponed until six weeks after delivery, as this is associated with high rates of perforation and expulsion. There are no doubts against breast feeding at lying IUD.
The available experience indicates that, in general, drugs interfering with contraceptive efficacy of Mona Lisa IUDs are highly unlikely. However, published reports appear to show diminished efficacy in the presence of long-term use of non-steroidal anti-inflammatory drugs (especially acetylsalicylic acid) and corticoids. Short-term use in the treatment of dysmenorrhoea with not-steroidal anti-inflammatory drugs does not appear to reduce contraceptive efficacy.
Mona Lisa IUDs may not be used at:
- Malignant diseases of the genital tract
- Vaginal bleeding
- Pregnancy
- Past history of ectopic pregnancy or predisposing factors
- infections of the genital tract
- Sexually transmitted diseases during the last 12 months (except bacterial vaginitis, repeated herpes infection, hepatitis B)
- Abortion with infection during the last 3 months, pelvic inflammatory disease (PID)
- Uterine malformations (congenital or acquired)
- Allergy to copper
MR Conditional
Non-clinical testing has demonstrated that the Mona Lisa intrauterine devices are MR condition. A patient with a Mona Lisa intrauterine device can be safely scanned in a MR system with a static magnetic field up to 3 Tesla.
The signed Declaration of the manufacturer is available under section "Quality System".

